
Back Calciumhexacyanidoferrat(II) German Kalsiumferrosyanidi Finnish Kalcium-ferrocianid Hungarian Гексацианоферрат(II) кальция Russian கால்சியம் பெரோசயனைடு Tamil
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.034.085 |
| EC Number |
|
| E number | E538 (acidity regulators, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6Ca2FeN6 | |
| Molar mass | 292.109 g·mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P332+P313 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
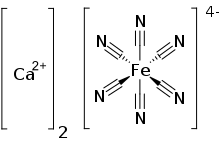
Calcium ferrocyanide is an inorganic compound with the formula Ca2[Fe(CN)6]. It is the Ca2+ salt of [Fe(CN)6]4-, ferrocyanide complex ion. A yellow solid, it is used as a precursor to the pigment Prussian blue.[1]
- ^ Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N.; Hasenpusch, W. (2011). "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub3. ISBN 978-3527306732.
